During the past EHA conference in Madrid, Eli Lilly hosted a satellite symposium on navigating the journey for patients with mantle cell lymphoma (MCL). During the symposium, Prof. Steven Gouill (Institut Paris, France), Dr. Ana Marin-Niebla (Vall d’Hebron Institute of Oncology, Barcelona, Spain) and Dr. Toby A. Eyre (NHS Foundation Trust, Oxford, UK) shared their knowledge and insights on considerations for early lines of treatment, as well as approaches following covalent Bruton’s tyrosine kinase inhibitors (BTKis).
Mantle cell lymphoma (MCL) is an aggressive, rare subtype of non-Hodgkin’s lymphoma. The initial treatment decision for a MCL patient is mainly dictated by the patient’s age and fitness. As such, the current standard of care (SOC) for younger, fit MCL patients is high-dose cytarabine-containing immunochemotherapy, followed by autologous stem cell transplantation (ASCT) and rituximab maintenance.1,2 For patients who are not fit for ASCT there is no generally accepted front-line therapy, but rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) is commonly used. Patients who are not fit enough for either of these approaches receive alternative therapies such as rituximab-bendamustine, rituximab-chlorambucil or rituximab plus cyclophosphamide, vincristine, and prednisolone (R-CVP).1,2
However, recent studies investigated other treatment combinations to further improve existing outcomes with these standard therapies. First of all, the phase III randomised, open-label, TRIANGLE trial investigated ibrutinib combined with immunochemotherapy with or without ASCT versus immunochemotherapy and ASCT in previously untreated, young patients with MCL (aged 65 years or younger). After 31 months median follow-up, the addition of ibrutinib to SOC proved to be superior to SOC with 3-year failure-free survival of 88% vs. 72% (p= 0.0008). On the other hand, superiority of standard treatment with ASCT over treatment with ibrutinib but without ASCT was not shown, with 3-year failure-free survival 72% vs. 86% (p= 0.9979). As such, the addition of ibrutinib during induction and as maintenance could become part of first-line treatment of younger MCL patients, but there is currently no decision on whether ASCT adds to ibrutinib.3
Furthermore, the randomised, double-blind, phase III SHINE study enrolled 523 previously untreated patients with stage II-IV MCL who were ineligible for ASCT and were aged over 65 years. Patients were randomised 1:1 to receive either ibrutinib or placebo until progressive disease or unacceptable toxicity. All patients also received induction with bendamustine and rituximab (BR) followed by rituximab maintenance in case of complete or partial response.4 In this study, a significant improvement in PFS was seen in patients who had received ibrutinib vs. placebo (median PFS: 6.7 vs. 4.4 years), representing a 50% improvement compared to placebo plus BR and R maintenance. However, no OS benefit was noted. As such, the inclusion of BTKis and the omission of ASCT in first remission might change the future front-line management of MCL.
For patients in first relapse or those who are primary refractory, a covalent BTKi should be offered. Ibrutinib monotherapy is considered as an approved and reimbursed standard of care option at first relapse.5 For BTKi exposed patients, we can consider CAR-T cells for fit patients (if available) or can use a reversible non-covalent BTKi (e.g. pirtobrutinib), an allogeneic stem cell transplantation or clinical trials with combinations of novel agents such as bispecific agents or BCL2 inhibitors.2
Recently, the phase I/II BRUIN study reported promising efficacy of the reversible BTK inhibitor pirtobrutinib in 152 patients with an ECOG of ≤2 who had received prior covalent BTKi treatment.6 In this population, an overall response rate of 49.3% was achieved, with a complete response seen in 15.8%. At a median follow up of 15.9 months, the median PFS was 5.6 months. The median time to first response was 1.8 months and after a median follow-up of 14.7 months, the median duration of response was 21.6 months. After a median follow-up of 24.2 months, median OS was 23.5 months. Median time on treatment was 5.5 months for the MCL cohort. Overall, 5% had treatment-related AEs leading to dose reductions and 3% had treatment-related AEs leading to pirtobrutinib discontinuation.6 Data from subgroup analyses revealed that results were consistent, including in high-risk subgroups and in patients who previously discontinued their prior BTKi due to toxicity.6 At present, the non-covalent BTKi pirtobrutinib has EMA approval for use as a monotherapy for adult patients with relapsed/refractory MCL who have previously been treated with a prior BTKi.7
Further, based on the results of the ZUMA-2 study, CAR-T cell therapy with brexucabtagene autoleucel is EMA-approved for use in adult patients with relapsed/refractory MCL following 2 or more lines of systemic therapy, including a BTKi. In this study, the objective response rate in the all-treated population was 91%, with a complete response rate of 68%. However, apart from logistical challenges, one of the potential issues of CAR-T cell therapy is its toxicity profile, with relatively high rates of grade ≥3 cytokine release syndrome (15%) and grade ≥3 neurologic events (33%).8,9
A male patient, aged 69 years old presented in 2014 with stage IV MCL with bone marrow, nodal and splenic involvement. The patient had a High MIPI score and his TP53 status was unknown. In March 2014, six cycles of R-CHOP, followed by rituximab maintenance were initiated (completed in January 2016). In March 2019, progressive disease with increasing superior mediastinal soft tissue mass and right axillary node involvement was visible on CT scan. In June 2019, the patient was enrolled in the FUSION NHL trial. Under ibrutinib, the patient achieved complete remission in September 2019 but experienced atrial fibrillation as of December 2020. After more than 4 years on ibrutinib, in September 2022 (78 years old) the patient progressed with widespread lymphadenopathy. At the time of progression, the patient had atrial fibrillation and hypertension on ibrutinib and repeat biopsy demonstrated classical histology, a Ki67 of 10-20% and mutated TP53. The patient was then treated with pirtobrutinib 200 mg. Under treatment with pirtobrutinib, the patient obtained a partial response with a 91.4% SPD reduction after 4 treatment cycles (Figure 1). Grade 3 neutropenia was the only treatment-related adverse event.* No infections were reported and the patient’s neutropenia was reversible with granulocyte colony-stimulating factor twice weekly. No dose interruption or cessations were necessary and the patient experienced no worsening of atrial fibrillation or hypertension. The patient had an excellent quality of life but eventually progressed after 15 months on pirtobrutinib.
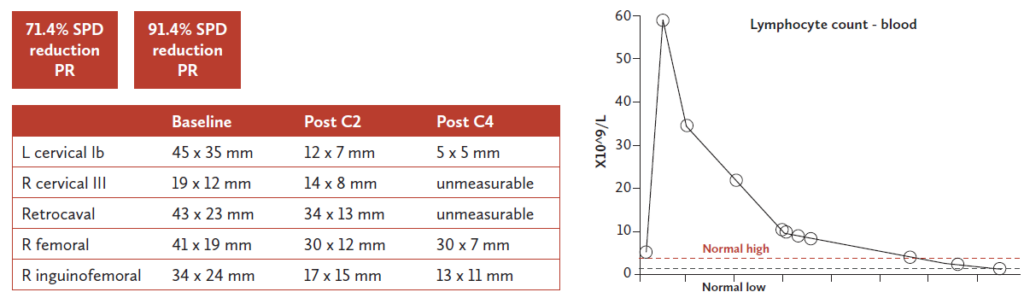
Figure 1. Patient case – response to pirtobrutinib treatment. Courtesy of Dr. Eyre.
To date, MCL is still an incurable disease but there has definitely been progress in improving treatment options. Especially the BTKis are gaining a prominent place in the treatment algorithm, with the use of ibrutinib in first-line and the use of pirtobrutinib in the relapsed/refractory setting for patients who previously received a covalent BTKi.
References
PP-PT-BE-0072 – September 2024
Pirtobrutinib is indicated in monotherapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL) who have been previously treated with a Bruton’s tyrosine kinase (BTK) inhibitor. This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Delivery mode: Medicinal product subject to restricted medical prescription. This material is meant only for individuals allowed by law to prescribe or deliver medicines. Copyright 2024 RP: Eli Lilly Benelux – Markiesstraat 1/4B Rue du Marquis, 1000 Brussel/Bruxelles
*This AE was reported according to the procedures of the clinical trial in which the patient was enrolled and treated.
| Price under negotiation |
MINIMAL INFORMATIONS OF THE SPC ▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions. 1. NAME OF THE MEDICINAL PRODUCT Jaypirca 50 mg filmcoated tablets Jaypirca 100 mg filmcoated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Jaypirca 50 mg filmcoated tablets Each filmcoated tablet contains 50 mg of pirtobrutinib. Excipients with known effect Each filmcoated tablet contains 38 mg of lactose (as monohydrate). Jaypirca 100 mg filmcoated tablets Each film-coated tablet contains 100 mg of pirtobrutinib. Excipients with known effect Each film-coated tablet contains 77 mg of lactose (as monohydrate). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Filmcoated tablet (tablet). Jaypirca 50 mg filmcoated tablets Blue, 9 x 9 mm, arctriangle shaped tablet debossed with “Lilly 50” on one side and “6902” on the other side. Jaypirca 100 mg filmcoated tablets Blue, 10 mm, round tablet debossed with “Lilly 100” on one side and “7026” on the other side. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Jaypirca as monotherapy is indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL) who have been previously treated with a Bruton’s tyrosine kinase (BTK) inhibitor. 4.2 Posology and method of administration Jaypirca therapy should be initiated and supervised by physicians experienced in the use of anticancer therapies. Posology The recommended dose is 200 mg pirtobrutinib once daily (QD). Jaypirca dosing should be interrupted until recovery to Grade 1 or baseline when the patient experiences the following event: Grade 3 neutropenia with fever and/or infection Grade 4 neutropenia lasting ≥ 7 days Grade 3 thrombocytopenia with bleeding Grade 4 thrombocytopenia Grade 3 or 4 nonhaematologic toxicity Asymptomatic lymphocytosis is not regarded as an adverse reaction, and patients experiencing this event should continue taking Jaypirca. In the clinical study, adverse events in a limited number of patients were managed by dose reduction (see section 5.1). Treatment should be continued until disease progression or unacceptable toxicity. Missed dose If more than 12 hours have passed after a patient has missed a dose, the patient should be instructed to take the next dose at its scheduled time; an additional dose should not be taken. If vomiting occurs, the patient should not take an additional dose but continue with the next scheduled dose. Special populations Elderly No dose adjustment is required based on age (see section 5.2). Renal impairment No dose adjustment is required for patients with mild, moderate or severe renal impairment. There are no data in patients on dialysis (see section 5.2). Hepatic impairment No dose adjustment is required for patients with mild, moderate, or severe hepatic impairment (see section 5.2). Paediatric population The safety and efficacy of Jaypirca in children and adolescents aged less than 18 years have not been established. No data are available. Method of administration Jaypirca is for oral use. The tablet should be swallowed whole with a glass of water to ensure consistent performance (patients should not chew, crush, or split tablets before swallowing) and can be taken with or without food. Patients should take the dose at approximately the same time every day. 4.3 Contraindications Hypersensitivity to the active substance or to any of the excipients listed in section 6.1. 4.8 Undesirable effects Summary of the safety profile The most common adverse reactions of any grade are: fatigue (26.3 %), neutropenia (22.8 %), diarrhoea (22.1 %) and contusion (19.0 %). The most common severe (Grade ≥ 3) adverse reactions are: neutropenia (19.7 %), anaemia (7.9 %) and thrombocytopenia (6.6 %). The frequency of treatment discontinuation due to adverse reactions is 1.2 % and the frequency of dose reductions due to adverse reactions is 3.3 %. The most common adverse reactions (reported in more than 2 patients) leading to dose reduction are neutropenia (1.8 %), fatigue (0.4 %), thrombocytopenia (0.3 %), anaemia (0.3 %) and rash (0.3 %). The most common adverse reactions (reported in more than 2 patients) leading to dose discontinuation are neutropenia (0.4 %) and pneumonia (0.3 %). Serious adverse reactions associated with Jaypirca have occurred in 11.3 % of patients and the most common serious adverse reactions (occurring in ≥1 % of patients) were pneumonia (4.7 %), neutropenia (2.2 %), anaemia (1.7 %) and urinary tract infection (1.0 %). Fatal adverse reactions have been observed in 0.3 % of patients (2 patients) for pneumonia and in 0.1 % of patients (1 patient) for haemorrhage. Tabulated list of adverse reactions Table 1 lists the adverse drug reactions (ADRs) associated with Jaypirca used as a monotherapy from clinical study data. The ADRs are based on pooled data from 583 patients treated with Jaypirca monotherapy 200 mg QD starting dose with no dose escalation in a phase 1/2 clinical study. Patients were treated for MCL, chronic lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL) and other non-Hodgkin lymphoma (NHL). Patients were exposed to Jaypirca for a median duration of 8 months. ADRs are listed below by MedDRA body system organ class. Frequency groups are defined by the following convention: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1 000 to < 1/100); rare (≥ 1/10 000 to < 1/1 000); very rare (< 1/10 000), and not known (cannot be estimated from the available data). Within each frequency grouping, ADRs are presented in order of decreasing seriousness. Table 1: ADRs of patients treated with Jaypirca monotherapya at 200 mg QD
| System organ class (MedDRA) | ADR | Frequency category (%) (All grades) | Grade ≥ 3c (%) |
| Infections and infestations | Pneumonia | Common (8.2) | 5.1 |
| Urinary tract infection | Common (6.9) | 0.7 | |
| Upper respiratory tract infection | Common (5.0) | 0 | |
| Blood and lymphatic system disorders | Neutropeniab | Very common (22.1) | 19.2 |
| Thrombocytopeniab | Very common (12.9) | 7.0 | |
| Anaemiab | Very common (14.4) | 8.2 | |
| Lymphocytosisb | Common (5.1) | 3.1 | |
| Nervous system disorders | Headache | Common (9.8) | 0.3 |
| Cardiac disorders | Atrial fibrillation/atrial flutter | Common (2.7) | 1.0 |
| Vascular disorders | Haemorrhageb | Very common (16.8) | 2.4 |
| Haematuria | Common (3.1) | 0.0 | |
| Epistaxis | Common (3.8) | 0.2 | |
| Haematoma | Common (1.9) | 0.2 | |
| Bruising | Very common ( 21.8) | ||
| Contusion | Very common (18.2) | ||
| Petechiae | Common (4.6) | ||
| Gastrointestinal disorders | Diarrhoea | Very common (19.9) | 0.9 |
| Abdominal pain | Very common (10.3) | 1.0 | |
| Nausea | Very common (14.1) | ||
| Skin and subcutaneous tissue disorders | Rashb | Very common (11.7) | 0.3 |
| Musculoskeletal and connective tissue disorders | Arthralgia | Very common (12.2) | 0.5 |
| General disorders and administration site conditions | Fatigue | Very common (23.7) | 1.2 |
a Frequencies are derived from Jaypirca exposure in patients with B-cell malignancies b Includes multiple adverse reaction terms c Severity grade assignment based on National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5.0 Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via: Belgium : Agence fédérale des médicaments et des produits de santé, www.afmps.be, Division Vigilance: Site internet: www.notifieruneffetindesirable.be, e-mail: adr@fagg-afmps.be. Luxembourg : Centre Régional de Pharmacovigilance de Nancy ou Division de la pharmacie et des médicaments de la Direction de la santé Site internet : www.guichet.lu/pharmacovigilance. 7. MARKETING AUTHORISATION HOLDER Eli Lilly Nederland B.V. Papendorpseweg 83 3528 BJ Utrecht The Netherlands 8. MARKETING AUTHORISATION NUMBER(S) EU/1/23/1738/001 EU/1/23/1738/002 EU/1/23/1738/003 EU/1/23/1738/004 EU/1/23/1738/005 EU/1/23/1738/006 EU/1/23/1738/007 EU/1/23/1738/008 EU/1/23/1738/009 9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION Date of first authorisation: 30 October 2023 10. DATE OF REVISION OF THE TEXT 16 August 2024. Detailed information on this medicinal product is available on the website of the European Medicines Agency http://www.ema.europa.eu. METHOD OF DELIVERY Medicinal product subject to restricted medical prescription.