The treatment landscape for patients with chronic lymphocytic leukaemia (CLL) has advanced significantly with the use of Bruton’s tyrosine kinase (BTK) inhibitors (BTKi) and B-cell lymphoma-2 (BCL2) inhibitors, particularly in first-line triple combination treatments. For example, the phase II GAIA/CLL13 trial showed high undetectable measurable residual disease (uMRD) rates and prolonged progression-free survival (PFS) in the first-line setting. However, evidence for patients with relapsed/refractory CLL remains limited, especially for patients who were previously exposed to BTKi and/or venetoclax. Therefore, the phase II CLL2-BZAG trial assessed an MRD-guided triple combination treatment of venetoclax, zanubrutinib, and obinutuzumab after optional bendamustine debulking, aiming to provide effective, time-limited therapy for patients with relapsed/refractory CLL.
The phase II investigator-initiated CLL2-BZAG trial enrolled patients aged >18 years with active and previously treated CLL. Exclusion criteria included disease progression during prior BTKi and/or venetoclax treatments, or patients with potential resistance-conferring mutations in BTK and/or BCL2. Patients at increased risk for tumour lysis syndrome received optional two cycles of bendamustine debulking. Induction treatment started with one cycle of obinutuzumab monotherapy, followed by the addition of zanubrutinib in the second cycle, which continued until the end of the study treatment. In the third induction cycle, venetoclax was introduced according to the established 5-week ramp-up schedule (Figure 1). In total, patients received 8 cycles of induction treatment (of which 6 with the triplet regimen) before they reached the final restaging. Maintenance therapy with obinutuzumab (1000 mg every 3 months), zanubrutinib (160 mg twice daily), and venetoclax (400 mg daily) was initiated after restaging. Maintenance treatment was continued until achieving complete response (CR) or clinical CR in combination with uMRD in the PB in two consecutive measurements or up to a maximum of 24 months.. MRD was analysed by flow cytometry, with uMRD defined as <1 CLL cell in 10.000 leukocytes. Circulating tumour DNA (ctDNA) mutations, including resistance markers, were assessed by digital droplet PCR (ddPCR). MRD and ctDNA were monitored monthly during induction and 3-monthly during maintenance. Bone marrow evaluation was mandatory to establish CR/CR with incomplete marrow recovery (CRi). The primary endpoint was the rate of patients with uMRD in the peripheral blood (PB) after six cycles of the full triple combination, assessed at final restaging after eight cycles of induction treatment. Secondary endpoints included overall response rate, CR rate, uMRD at different time points, PFS, overall survival (OS), and safety.
A total of 42 patients (median age 64 years) were enrolled, with two excluded from the analysis population due to receiving <2 induction cycles. Patients had a median of one prior therapy (range 1-5). Of them, 45% had received a BTKi, 17.5% venetoclax; and, of these, 12.5% had received both. TP53 mutations/deletions were present in 37.5% of the patients and 77.5% had unmutated immunoglobulin heavy chain variable region gene. After a median follow-up of 21.5 months, all patients responded at the final restaging, with 7.5% achieving a CR or CRi and 92.5% achieving partial response. In total, 52.5% of patients achieved uMRD in the PB at the final restaging. Interestingly, responses further deepened with ongoing treatment, reaching a best uMRD rate of 85%. The estimated PFS and OS rates at 18 months were 96% and 96.8%, respectively, with no patients requiring subsequent treatment. Best uMRD and survival rates were similar for patients previously exposed to BTKi and/or venetoclax and patients with TP53 aberrations. Bendamustine debulking was administered to 47.5% of the patients but was not associated with better efficacy outcomes in this trial. The most common adverse events of all grades included COVID-19 (61.9%), diarrhoea (35.7%), infusion-related reactions (35.7%), thrombocytopenia (33.3%), nausea (28.6%), fatigue (28.6%), and neutropenia (28.6%). In total, 18% of adverse events led to an adjustment of at least one study drug. Two patients had fatal adverse events related to COVID-19 and fungal pneumonia secondary to COVID-19.
The MRD-guided triple combination of zanubrutinib, venetoclax, and obinutuzumab in the CLL2-BZAG study showed promising efficacy, including deep remissions, in patients with relapsed/refractory CLL, including those with high-risk genetics and prior exposure to BTKi/venetoclax.
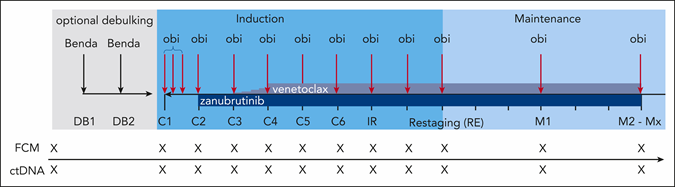
Figure 1. Treatment and sampling schedule1. Every “X” on the sampling schedule represents an FCM and ctDNA sample. Benda= bendamustine; C= cycle; DB= debulking; FCM= flow cytometry; IR= initial response assessment; M= maintenance staging; Mx= maintenance stagings 3 to 8; obi= obinutuzumab; RE= final restaging.